What Is The Half Life Of An Isotope . As the temperature of a sample of a radioactive element decreases, the half. The time taken for the radioactivity of a specified isotope to fall to half its original value.
Machines at War 3 from www.macgamestore.com
In this experiment, you will use a source called an isogenerator to produce a sample of radioactive barium. A few hours, most of the radioactivity will be gone in a few days. 113 rows wikimedia list article.
Machines at War 3
What is the formula for half life? Radioactive isotopes are used for blood flow monitoring, cancer treatment, paper mills, carbon dating and smoke alarms. 113 rows wikimedia list article. So as you can see from these pictures, it really depends on what variable or information you need to find.
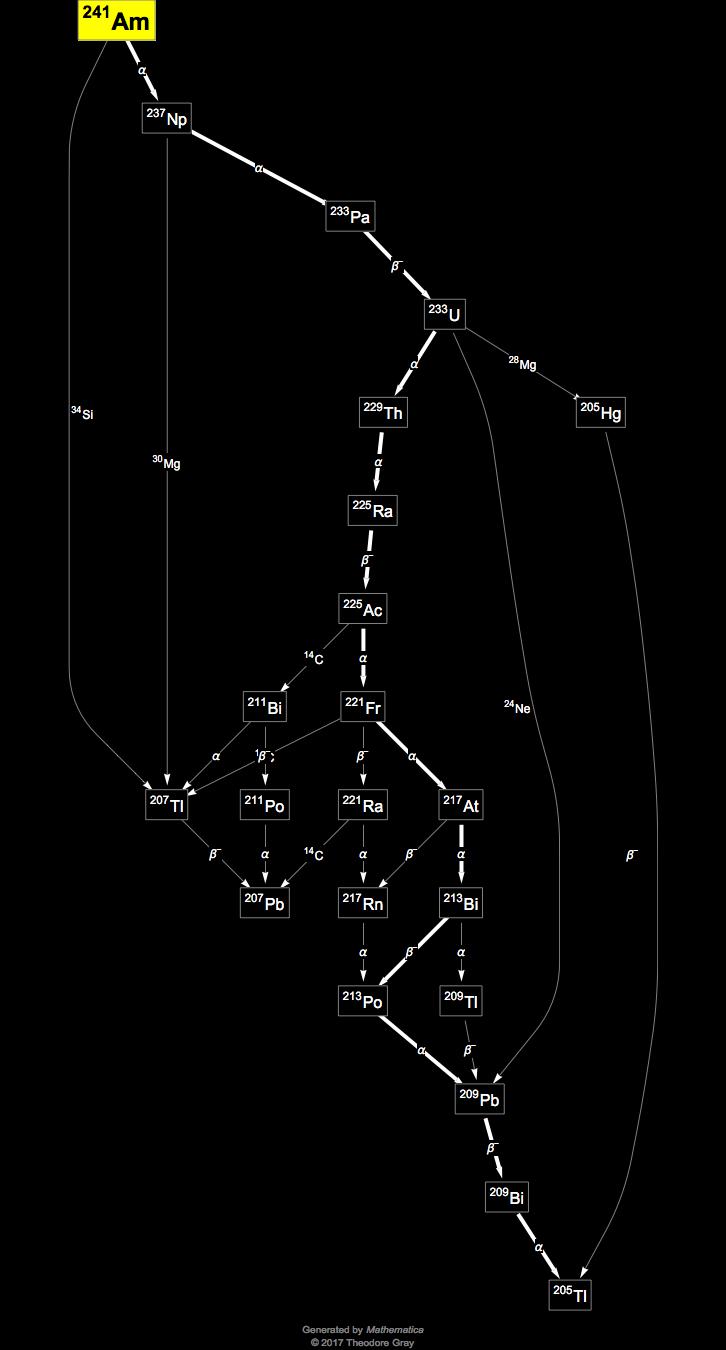
Source: periodictable.com
Check Details
Which fraction represents the amount of an original sample of this element remaining after 6 days? 80 years, it will take a long time for significant decay to occur. What is the formula for half life? A few hours, most of the radioactivity will be gone in a few days. As the temperature of a sample of a radioactive element.
Source: periodictable.com
Check Details
Which fraction represents the amount of an original sample of this element remaining after 6 days? It is unaffected by conditions and is independent of the initial amount of that isotope.jul 31, 2021 As was written, radioactive decay is a random process at the level of single atoms, in that, according to quantum theory, it is impossible to predict when..
Source: www.macgamestore.com
Check Details
As radioactive isotopes of elements decay, they lose their radioactivity and become a brand new element known as a daughter isotope. It measures the time it takes for a given amount of the substance to become reduced by half as a consequence of decay, and therefore, the emission of radiation. The time taken for the radioactivity of a specified isotope.
Source: www.slideshare.net
Check Details
80 years, it will take a long time for significant decay to occur. The initial amount of radioactive isotope present= 5 g. As the temperature of a sample of a radioactive element decreases, the half. A few hours, most of the radioactivity will be gone in a few days. What is the half life of an isotope?
Source: www.slideshare.net
Check Details
Radioactive isotopes are used for blood flow monitoring, cancer treatment, paper mills, carbon dating and smoke alarms. Which fraction represents the amount of an original sample of this element remaining after 6 days? A half life of a radioactive substance is. Similarly, the elapsed time t and the initial quantity n (0) of a radioactive isotope can also be calculated.
Source: www.sciencephoto.com
Check Details
Radioactive isotopes are used for blood flow monitoring, cancer treatment, paper mills, carbon dating and smoke alarms. In this experiment, you will use a source called an isogenerator to produce a sample of radioactive barium. The time taken for the radioactivity of a specified isotope to fall to half its original value. 80 years, it will take a long time.
Source: www.writework.com
Check Details
In this experiment, you will use a source called an isogenerator to produce a sample of radioactive barium. 80 years, it will take a long time for significant decay to occur. So as you can see from these pictures, it really depends on what variable or information you need to find. The time taken for the radioactivity of a specified.